|
|||||||||||||
|
|||||||||||||
cross-sections of samples were examined by the optical microscope MMR-4 and scanning electron microscopy JEOL. The corrosion properties of the magnets (See Table 3) were investigated under the complete immersion of the samples in the naturally aerated 1% sodium sulphate solution. The polarisation of electrodes was carried out by the potentiostate PI-50-1 and programmator PR-8 under the controlled potential regime by the three-electrode scheme. A three-electrode cell arrangement was used for the electrochemical measurements, with platinum needle-like and remote saturated chloride-argentums half-cell (the potential of 0.201 V) as auxiliary and reference electrodes respectively. The reference electrode was connected with the cell via electrolytic switch. 3 Results and Discussion3.1 Sm-Co + Ti coatingOne of the main requirements for the protective coatings, aimed to resist on corrosion, is the absence of pores. The pores can provide contact between aggressive environment and the surface of protected sample. Although the condensate which deposited from separated plasma stream under vacuum-arc ion-plasma deposition has low grain size (<1.5 ?m), the number of open pores per unit area can reach critical level even for the coating with the thickness of 20 ?m [8]. Moreover, such coatings have very non-equilibrium structure with the high tendency to aging. The brittleness of such coatings is increased during aging process which leads to the cracking of the coating and results in the deterioration of magnetic properties. All these drawbacks are expected to be overcome by the following treatment of the coatings with pulsed plasma streams. It should be noted that the thickness of preliminary coating and the regime of plasma treatment has to be accurately adjusted and controlled. Fig. 1 shows the surface of Sm-Co magnet with titanium coating (thickness of 50 microns) after helium plasma treatment. The melting of the titanium coating and the healing of open pores took place as a result of helium plasma treatment with energy density of 28 J/cm2. Despite favorable healing of pores, the surface has a complex and non-homogeneous structure resembling streams of solidified metal. This shape of the surface can be evidence for the liquid state of the titanium coating under plasma treatment. Partial reflow of the Ti coating takes place on the flanks of the sample, but the area of thermal reflow is not uniform.
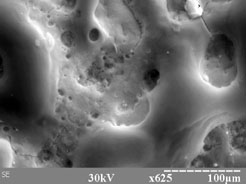

The cross-section of plasma treated Sm-Co magnet with the titanium coating is shown on Fig 2. It can be seen that as a result of the plasma treatment modified fine-grained layer with the thickness of 50-70 microns has been formed. The bulk part of the sample has typical structure of sintered Sm-Co magnet. According to SEM analyses, the modified layer consists of pure titanium. It was observed that a considerable diffusion of titanium to the depth of 20 ?m took place. Table 2 shows the result of EDXF analysis of Sm-Co magnets with titanium coating after plasma treatment. The analysis was done on 20 ?m beneath the Ti layer. A high concentration of titanium (33.1 at.%) has been observed in the transitional mixed layer. Whereas the content of samarium, copper and iron decreased (See Table 2), the concentration of cobalt increased significantly. This effect can be attributed to the high sputtering coefficients of Sm, Cu and Fe. Such a transition mixed layer can be formed as a result of two complex processes. Firstly, because of anomalous diffusion of Ti atoms stimulated by the ion bombardments of the sample surface before the deposition of the titanium film, for the surface cleaning. And secondly, due to complicated processes which take place during interaction between plasma and solid material. It seems that both process give contribution to the final composition shift.
after pulsed plasma treatment 28 J/cm2 for 5 pulses. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Element | Integral content of the bulk magnet (20 microns) under modified layer at. % | Bulk content of Sm-Co magnet at. % |
| Sm | 9.529 | 13.104 |
| Co | 37.955 | 56.081 |
| Cu | 2.948 | 6.690 |
| Fe | 16.437 | 22.021 |
| Ti | 33.130 | - |
The optimization of plasma treatment was carried out by adjusting both energy loads and the thickness of preliminary titanium film. It was revealed that the higher the energy loads of plasma treatment the higher the roughness of the modified surface. While the flanks of the sample underwent remelting under high energy loads with energy density of 28 J/cm2, the decrease of the energy density to 25 J/cm2 was not favourable for the remelting of the large area of the flanks. The surface of treated area became non-homogeneous.
The considerable decrease of energy loads to 20 J/cm2 with simultaneous thinning of titanium film to 10 ?m resulted in the delamination of the coating under the plasma treatment. Titanium layer became non-solid and partly covered the surface of the magnet. Besides, the grid of cracks appeared which can be result of non-equilibrium thermal process during high-speed cooling of the modified layer and due to the difference in thermal-expansion coefficient between treated layer and bulk.
3.2 The corrosion properties of Nd-Fe-B magnets with ferroboron (Fe80B20 wt. %) coatings.
Table 3 shows the corrosion characteristics of various Nd-Fe-B samples in 1% sodium sulphate solution. The results are obtained from the potentiometric and polarization data for the set of experiments with the samples (See Table1). The potential of corrosion is scaled to the standard hydrogen electrode.
The polarization data shows that mechanical pre-treatment of initial sample leads to the decrease of corrosion resistance. As can be seen from the Table 3, non-polished sample 2 in comparison with polished sample 1 demonstrates the inhibition both anodic and cathode reactions of the corrosion process. Moreover, the inhibition of cathode reaction of corrosion process near the corrosion potential is higher ((dE/dI)к=3941) than for anodic reaction ((dE/dI)а=2901).
Table 3 The corrosion characteristics of various Nd-Fe-B
samples (See Table 1) in 1% NaCl solution
samples (See Table 1) in 1% NaCl solution
| № | Е corr,V | R p, Om | Tafel constant, V | I corr x104, А/dm2 | kh, mm/year | Fatigue point | |
| bа | bк | ||||||
| 1 | -0,528 | 2574 | 0,073 | 0,046 | 10,4 | 0,121 | 6 |
| 2 | -0,494 | 3421 | 0,065 | 0,051 | 7,2 | 0,085 | 5 |
| 3 | -0,538 | 3341 | 0,065 | 0,065 | 7,8 | 0,090 | 5 |
| 4 | -0,468 | 4184 | 0,061 | 0,068 | 6,8 | 0,079 | 5 |
| 5 | -0,497 | 4497 | 0,058 | 0,063 | 5,2 | 0,060 | 5 |
| 6 | -0,524 | 4281 | 0,059 | 0,055 | 5,9 | 0,069 | 5 |
| 7 | -0,530 | 2169 | 0,071 | 0,051 | 7,8 | 0,090 | 5 |
The values of Tafel constants of cathode (bk) and anodic (ba) reactions for samples 1 and 2 show that there is protective film of corrosion products on the surface of the samples which prevents oxygen supply and provides the barrier for the depolarizer. The currents of co/SUPrrosion and corrosion coefficients are strong evidence for it (See Table 3).
Fig. 3a shows the surface of polished sample 1 after corrosion test. The examination of polished and non-polished samples after anodic polarization allows to conclude that in all cases, the surfaces of the samples were covered with the sports of corrosion. However, polished sample 1 have higher level of failure with sports uniformly spread within the surface, than non-polished sample 2 which surface was covered with oxidizes of red-brown color.
It is believed that the rough surface with large and reactive surface area generated by plasma treatment of oxidized surface or previously deposited ferroboron coating can result in continuous layer with porous structure, which could eventually increase the bonding strength between the second ferroboron coating and the substrate.
The characteristics of corrosion process of plasma treated samples can be compared with the non-polished sample 2. An analysis of Tafel dependences demonstrates that the inhibition of cathode and anodic reactions for the samples 3, 4, 5, 6 is identical.
While sample 5 has thick layer of corrosion products of brown color, which block its surface and decrease the corrosion rate (See Fig. 3b), such a typical friable layer is absent on the surface of the sample 4.
Despite twice lower value of Rp for the sample 7 than for sample 6, which points to its lower corrosion resistance, Tafel constants show the less complication of cathode reactions of these samples and as a result increase of the general corrosion rate. Pre-processing of the sample 6 before plasma treatment in comparison with sample 7 promotes the raise of corrosion resistance (See Table 3). The potential of corrosion of these samples in comparison with the standard iron potential is appeared more electronegative. It can be attributed to the formation of neodymium compounds in the near-surface layers, despite the presence of protective ferroboron layer.
Continuous efforts to enhance the surface properties of the engineering components have resulted in the development of various processing techniques. Utility of high-power plasma processing to transform the easily amorphous ferroboron coatings to durable and firm coating is demonstrated.
Alloys with the lack of atomic long-range ordering have been always of interest for its mechanical, chemical, optical, thermal and electrical properties. The comparative analysis of various properties of crystal and amorphous alloys shows that amorphous alloys have higher strength, corrosion resistance, ductility and radiation resistance.
Since amorphous alloys are metastable systems, highly non-equilibrium conditions, which are among the main principles of amorphous alloys’ manufacturing, can be provided with high cooling rate of 106-108 K/s. Besides well-known technique of high-speed quenching such as vacuum-arc plasma deposition technique and laser treatment, pulsed plasma treatment is expected to be the challenging method. Despite high energy loads under plasma treatment, there was no desirable enhanced contact between the liquid phase and the base of the sample and the presence of the thin layer of absorbed gas additions, which also exists under conventional quenching techniques, introduced ruinous defects. Although cooling and heating rates of near-surface layers can be controlled via plasma parameters, accumulated gases can partly hinder the amorphisation.
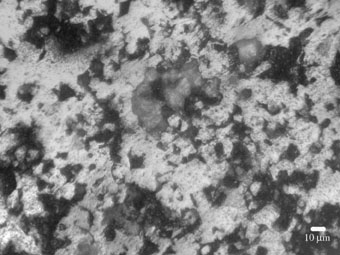
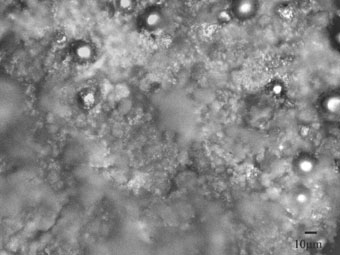
The primary test results listed in Table 3 show that the roughness of the samples caused by plasma treatment is one of the important factors to enhance the corrosion resistance due to the large and reactive surface area, which results in a strong mechanical interlocking and chemical bonding after the sealing of the samples with second ferroboron coating. The deposition of ferroboron on the plasma treated surfaces creates a porous and crack free amorphous film on the surface, which also helps to improve the bonding strength due to the penetration of the ferroboron coating into the porous plasma treated layer. However the non-polished samples with ferroboron coatings do not give a good bonding with the bulk of the magnet and modified layer after plasma treatment. One reason is the weak mechanical interlocking between the first coating layer and the substrate because of the presence of the thin and easily exfoliated oxide film on the surface. Another reason is the very non-uniform modified layer with cracks, which may leads to the weak bonding between the ferroboron coating layer and the substrate. Further experiments in improving the mechanical performance of the coating layer are under way.
It is well known that grain boundaries of crystal alloys are the nuclei of the facilitated corrosion. The structure of quickly cooled alloys consists of low-scale crystals which result in higher total surface area than in the slow cooled alloys. Thus high corrosion resistance of quickly quenched protective coatings in comparison with conventionally produced coatings shows that grain boundaries of the former have little influence on corrosion behavior due to the absence of chemical composition fluctuations promoting corrosion. Despite crystal state of quickly cooled alloys, it is expected that they do not have local fluctuation of chemical composition on the defects like grain boundaries and attribute to high chemical homogeneity. The existing structural defects induced by inappropriate plasma treatment like phase inhomogeneity, precipitations, segregations accompanied by chemical composition fluctuations may provide the sources of corrosion.
4 Conclusions
Pulsed plasma treatment of Ti-coated Sm-Co magnets creates a porous and crack free protective coating on the surface. This Ti-rich layer can improve the bonding strength due to the penetration of the titanium into the porous Sm-Co magnet structure. According to SEM analysis considerable diffusion of titanium on the depth of 20 microns took place. However solidness of the protective titanium layer is significantly depends on the thickness of the initial Ti film and plasma loads.
The corrosion properties of the Nd-Fe-B magnets vary through the pre-treatment procedure and plasma processing regimes. It was revealed that polishing of the initial samples before plasma treatment does not give a good bonding with ferroboron coating. The roughness of the sample caused by oxidizing and plasma processing is one of the important factors to enhance the bonding strength due to the large and reactive surface area, which could result in a strong mechanical interlocking and chemical bonding after ferroboron deposition.
It was observed that plasma treatment increases the polarization of the magnets. Samples can be ranked as follow
References:
[1] S. Trout, “Optimum corrosion protection of Nd-Fe-B magnets”, Proc. Advanced in Magnetic Application, Technology and Materials, Dayton, 2004.
[2] V.I.Tereshin, V.V. Chebotarev, A.M. Bovda, I. E. Garkusha, Nukleonika 2001 46(1): 27-30
[3] V.I. Tereshin, A.N. Bandura, I. E. Garkusha, I.G. Brown, O.V. Byrka, V.V. Chebotarev, A.S. Tortika, Review of Scientific Instruments, 2002, 73(2): 831-833
[4] V. L. Yakushin, Russian Metallurgy (Metally), 2005, 2 : 104-114
[5] I. E. Garkusha and et al, PLASMA 2005: Int. Conf. on Research and Applications of Plasmas; 3rd German-Polish Conf.on Plasma Diagnostics for Fusion and Applications; Opole-Turawa (Poland), 6-9 September 2005
[6] Sudzuki and et al., Amorphous Materials, Moscow: Metallurgy, 1987
[7] A.M.Bovda, V.A.Bovda, O.V.Byrka, V.V.Chebotarev, V.D.Fedorchenko, I.E.Garkusha, V.I.Tereshin. Advances in Applied Plasma Science, 2005, 15: 199-204.
[8] Leonov S. A., Belous V. A., Khorochikh V. M., Proc. Vacuum Technology and Equipment, Kharkiv, Ukraine, 1999, p. 25-29
|


